12 Nov Published! MTHFD2 and IMPDH2 metabolic enzymes in the nucleus
Nuclear metabolism for the win!
We are excited to publish two back-to-back manuscripts about nuclear metabolism. While the stories diverge into distinct concepts, it’s exciting to see metabolic enzymes do so much in the nucleus.
Read the original publication about MTHFD2 at Nature Communications.
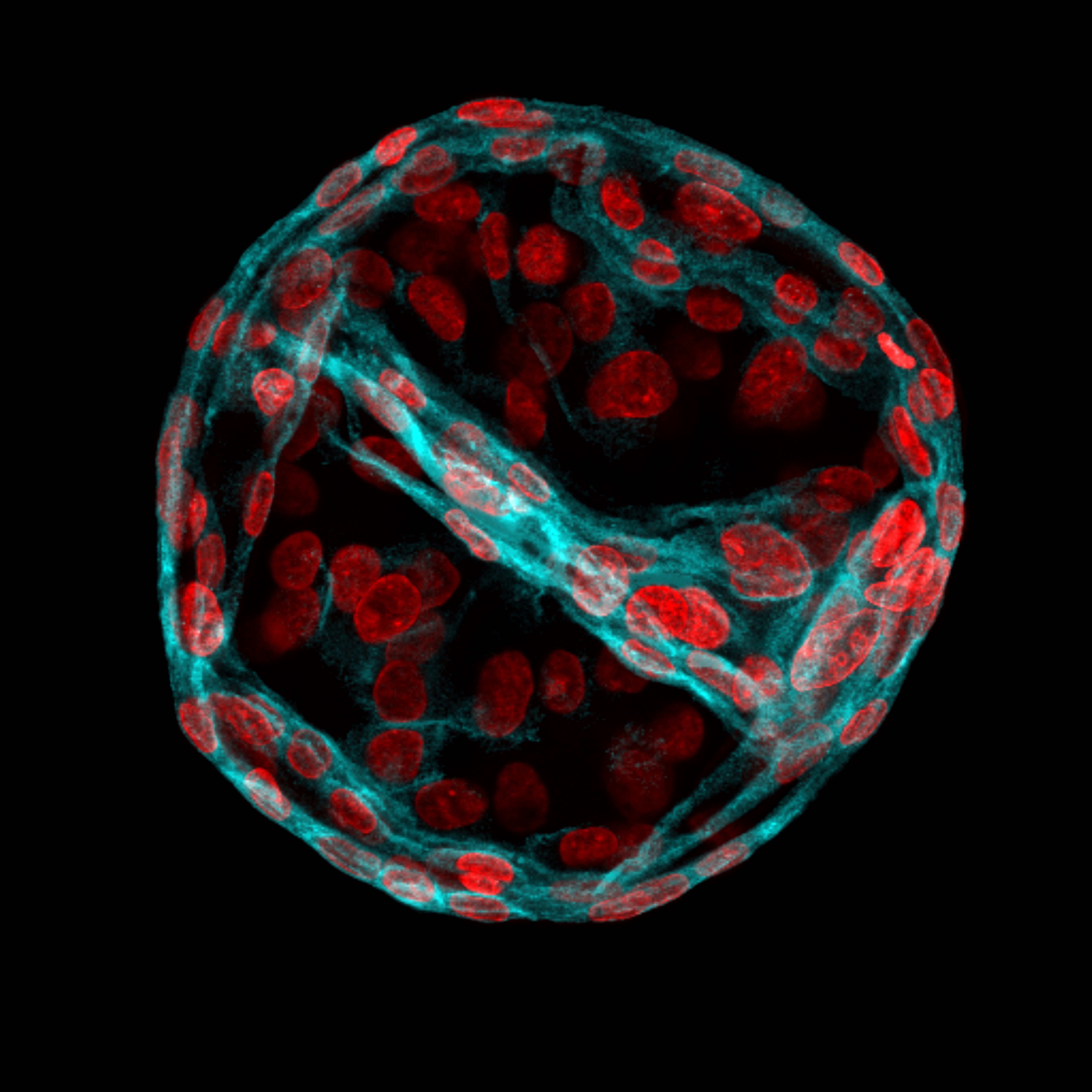
Nuclear localization of MTHFD2 is required for correct mitosis progression
We are excited to share the findings from our latest research, which uncovers a critical role for the mitochondrial enzyme MTHFD2 in ensuring proper cell division. Our study reveals that MTHFD2’s presence in the nucleus is essential for mitosis progression and chromosomal stability. We discovered that nuclear MTHFD2 interacts with key proteins responsible for regulating mitosis and maintaining centromere integrity, such as KMT5A and DNMT3B. Without MTHFD2, cells experience severe methylation defects, leading to errors in chromosome segregation and structural abnormalities.
Interestingly, we found that blocking the nuclear function of MTHFD2 replicates these defects, while directing MTHFD2 to the nucleus restores normal cell division. This groundbreaking research highlights the importance of metabolic enzymes in the nucleus, where they support critical chromatin processes, further advancing our understanding of cell cycle regulation and epigenetics.
Nuclear IMPDH2 controls the DNA damage response by modulating PARP1 activity
On the other hand, we find that nuclear metabolism and DNA damage response are intertwined processes. Here, we explore this crosstalk using triple-negative breast cancer (TNBC) as a model, a subtype often prone to DNA damage accumulation. We show that the de novo purine synthesis enzyme IMPDH2 is enriched on chromatin in TNBC compared to other subtypes. IMPDH2 chromatin localization is DNA damage dependent, and IMPDH2 repression leads to DNA damage accumulation. On chromatin, IMPDH2 interacts with and modulates PARP1 activity by controlling the nuclear availability of NAD+ to fine-tune the DNA damage response. However, when IMPDH2 is restricted to the nucleus, it depletes nuclear NAD+, leading to PARP1 cleavage and cell death. Our study identifies a non-canonical nuclear role for IMPDH2, acting as a convergence point of nuclear metabolism and DNA damage response.
Read the original publication about IMPDH2 at Nature Communications.




